Client Services: 800-959-2846|Billing: 949-374-501
UTIDX®

ABOUT OUR TEST
UTIDX® is a urine-based test designed to identify pathogens commonly associated with recurrent or persistent urinary tract infections while determining the best treatment options for the patient. Utilizing PCR technology, we are able to detect the presence of pathogens as well as the presence of antibiotic-resistant genes. PCR is paired with our internally validated methods for testing Antibiotic Sensitivity Testing of the identified pathogens to ensure personalized therapy options for the patient.
WHEN WAS THE LAST TIME YOUR PROVIDER UPDATED THEIR ANTIBIOTIC DATABASE?
As antibiotic usage recommendations evolve in response to new scientific discoveries, advances in technology, and changes in clinical practice, references such as CLSI ensure laboratory procedures and treatment recommendations remain current and effective. At EmeritusDX, our references are updated at least annually to keep up with current recommendations.
WHAT DOES UTIDX® COVER?
Our comprehensive test was developed to deliver results for even the most difficult UTIs to diagnose and treat. By targeting 31 uropathogens, 38 antibiotic resistant genes, and phenotypical evaluation of the 22 most recommended antibiotics for treatment, you can feel confident in getting to the right answer with UTIDX™.
OUR REQUISITION
Review our requisition for a complete list of the Urology services that we provide.
How TO interpret the UTIDX™ Report
Check out this great video that breaks down the power of our UTIDX™ Report
About the Bladder17™ bladder cancer test
Bladder cancer is one of the major cancers of the urinary system that is one of the most expensive cancers to treat due to a high recurrence rate.
Cystoscopy is often augmented by a non-invasive laboratory method such as urine cytology. Bladder17™ provides sensitivity and specificity to significantly improve the diagnosis and treatment of bladder cancer patients.
Pre-Prostate Biopsy Rectal Swab (PPBRS)

ABOUT OUR TEST
PPBRS is a rectal swab based test designed to identify the presence of pathogens commonly associated with infection post surgery. Utilizing PCR technology, we are able to detect the presence of pathogens as well as the presence of antibiotic resistant genes. PCR is paired with our internally validated methods for testing Antibiotic Sensitivity Testing of the identified pathogens to ensure personalized therapy options for the patient.
WHAT DOES PPBRS COVER?
Testing for the presence of pathogens and drug-resistant bacteria before prostate biopsies can help reduce infections. Our comprehensive test targets 31 pathogens, 28 antibiotic resistant genes, and provides phenotypical evaluation of the 27 most recommended antibiotics for treatment. With our PPBRS test, you can feel confident in lowering your risk or post-surgical infection.
OUR REQUISITION
Review our requisition for a complete list of the Urology services that we provide.
Prostatitis

ABOUT OUR TEST
Our Prostatitis test is a post-massage urine and/or semen based test designed to identify pathogens commonly associated with acute or chronic prostatitis while determining the best treatment options for the patient. Utilizing PCR technology, we are able to detect the presence of pathogens as well as the presence of antibiotic resistant genes. PCR is paired with our internally validated methods for testing Antibiotic Sensitivity Testing of the identified pathogens to ensure personalized therapy options for the patient.
WHEN WAS THE LAST TIME YOUR PROVIDER UPDATED THEIR ANTIBIOTIC DATABASE?
As antibiotic usage recommendations evolve in response to new scientific discoveries, advances in technology, and changes in clinical practice, references such as CLSI ensure laboratory procedures and treatment recommendations remain current and effective. At EmeritusDX, our references are updated at least annually to keep up with current recommendations.
WHAT DOES PROSTATITIS COVER?
Our comprehensive test was developed to deliver results for even the most difficult cases to diagnose and treat. By targeting 31 uropathogens, 38 antibiotic resistant genes, and phenotypical evaluation of the 22 most recommended antibiotics for treatment, you can feel confident in getting to the right answer.
OUR REQUISITION
Review our requisition for a complete list of the Urology services that we provide.
STIDX™

ABOUT OUR TEST
STIDX™ is a urine based test designed to identify pathogens commonly associated with sexually transmitted infections. Utilizing PCR technology, we are able to detect the presence of 8 pathogens. Our turnaround time for the test is 24 hours.
WHAT DOES OUR STI PANEL COVER?
Our comprehensive test was developed to deliver results for even the most difficult UTIs to diagnose and treat. By targeting pathogens, you can feel confident in getting to the right answer with our STI Panel.
OUR REQUISITION
Review our requisition for a complete list of the Urology services that we provide.
Urology Services
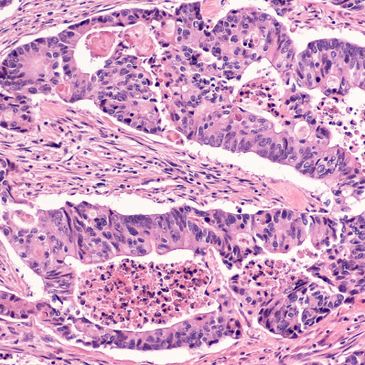
Prostate Biopsies
Prostate biopsies are performed when results from initial tests, like a prostate-specific antigen (PSA) blood test or digital rectal exam, indicate the possibility of prostate cancer. A range of tissue samples are extracted with a needle from the prostate gland and are analyzed under a microscope using a Hematoxylin & Eosin stain, and in
Prostate biopsies are performed when results from initial tests, like a prostate-specific antigen (PSA) blood test or digital rectal exam, indicate the possibility of prostate cancer. A range of tissue samples are extracted with a needle from the prostate gland and are analyzed under a microscope using a Hematoxylin & Eosin stain, and in some cases, immunohistochemical stains to identify cancerous cell abnormalities. If cancer is present, it can be localized to the exact region of the prostate from the location of the tissue extraction.

Bladder & Urovysion FISH
This FISH test is designed to detect aneuploidy for chromosomes 3, 7, and 17 and loss of the 9p21 locus, via fluorescence in situ hybridization (FISH) in urine specimens from persons with hematuria suspected of having bladder cancer. Results are intended for use in conjunction with, and not in lieu of, current standard diagnostic procedur
This FISH test is designed to detect aneuploidy for chromosomes 3, 7, and 17 and loss of the 9p21 locus, via fluorescence in situ hybridization (FISH) in urine specimens from persons with hematuria suspected of having bladder cancer. Results are intended for use in conjunction with, and not in lieu of, current standard diagnostic procedures (urine cytology) as an aid for initial diagnosis of bladder carcinoma in patients with hematuria and subsequent monitoring for tumor recurrence in patients previously diagnosed with bladder cancer.

UTIDX™
Urine Cytology
UTIDX™ is a urine based test designed to identify pathogens commonly associated with recurrent or persistent urinary tract infections while determining the best treatment options for the patient. Utilizing PCR technology, we are able to detect the presence of pathogens as well as the presence of antibiotic resistant genes. PCR is paire
UTIDX™ is a urine based test designed to identify pathogens commonly associated with recurrent or persistent urinary tract infections while determining the best treatment options for the patient. Utilizing PCR technology, we are able to detect the presence of pathogens as well as the presence of antibiotic resistant genes. PCR is paired with our internally validated methods for testing Antibiotic Sensitivity Testing of the identified pathogens to ensure personalized therapy options for the patient.

Urine Cytology
Urine Cytology
Urine Cytology
Urine cytology is a test to look for abnormal cells in your urine. It's used with other tests and procedures to diagnose urinary tract cancers, most often bladder cancer. A urine cytology test may be initiated if blood is detected in the urine (hematuria). For people who've been diagnosed with bladder cancer and have undergone treatment, a urine cytology test can help detect a recurrence

Bladder17™
Urine Cytology
Bladder17™
Uro17™ is a urine-based biomarker test to detect bladder cancer in people with symptoms of bladder cancer. It is also used to monitor for recurrence during treatment follow up. The innovative aspect is that it is a non-invasive diagnostic tool that detects bladder cancer based on the novel biomarker keratin 17 (K17). It can help stratify
Uro17™ is a urine-based biomarker test to detect bladder cancer in people with symptoms of bladder cancer. It is also used to monitor for recurrence during treatment follow up. The innovative aspect is that it is a non-invasive diagnostic tool that detects bladder cancer based on the novel biomarker keratin 17 (K17). It can help stratify patients with suspected bladder cancer and prioritize them for further secondary care investigations
UROLOGY REQUISITION
Review our requisition for a complete list of the Urology services that we provide
Copyright © 2026 Emeritus Medical Technology - All Rights Reserved.
